
Integrated Continuous Downstream Processes for Next-generation of Biologics
Integrated continuous downstream processes are considered to be an important part of the next generation production system because of higher productivity and smaller foot print. The project proposal is divided in four work packages which are centred round three industrial case studies.
Integrated continuous downstream processes are considered to be an important part of the next generation production system for new biologics. Integrated continuous downstream processes have higher productivity and smaller foot print compared to existing processes. The introduction of a new processing paradigm in the biopharmaceutical industry requires methods and tools for process development and verification in all stages in the drug candidate development. This proposal will add design and control tools for increased flexibility and controllability of the process at development in both preclinical stage and in clinical studies. The project proposal is divided in four work packages which are centred round three industrial case studies to give the result maximum of impact in the industry. Industrial partners are Novo Nordisk, Sobi and Modelon. The project is funded by Vinnova under the Next-generation Biologics initiative.
The main deliverables are the following:
- A design of flexible multi column single setup system for integrated continuous DSP for preclinical studies
- Design studies on integrated continuous DSP for clinical studies and commercial production
- A design of flexible reactor-separator configuration in integrated continuous DSP for protein modification
- An external supervisory controller for multiple steps and multiple setups for integrated DSP
- Engineering tools and methods for the development of integrated continuous DSP
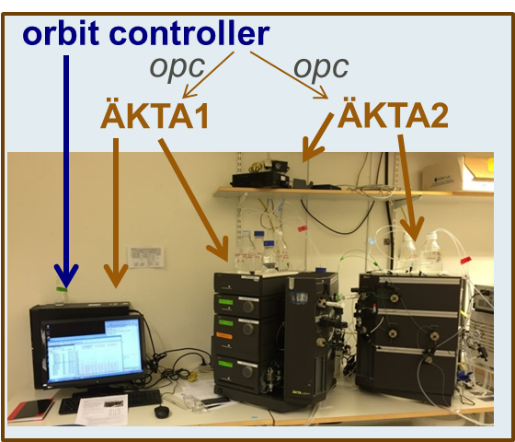
A twin-column setup for continuous chromatography with recycle for optimal performance with high purity and high yield.