PiiA-bio: Control and Automation of Industrial Biological Processes
The aim with the PiiA-bio project is to develop solutions to increase productivity in biotechnology and biopharmaceutical industry through increased production optimization and automation while maintaining high product quality. The project ended in april 2017.
Biotechnology in general, and the biopharmaceutical industry in particular, has a low level of automation, as well as high or exceptionally high quality standards. This often results in overly robust processes with a low productivity. The industry has a general demand for small-scale production, often with severe time constraints, for pre-studies of new products. It is therefore of utmost interest to the industry to develop production platforms with the ability to relatively autonomously produce small batches of pure protein.
The project focuses on the two main stages of a biotechnological process: fed-batch reactor and industrial chromatographic separation. The aim is to develop unique solutions for optimal control of the two stages, which both have a great innovative potential. A third part of the project is focused on developing a production platform for laboratory scale production. Here, technology and expertise from large-scale production is moved down to the early research and development labs. This aims to meet the industry demand for small-scale, fast and autonomous production. Other than Lund University, involved actors are Novo Nordisk, Pfizer Health and Novozymes. The project is co-financed by the involved actors and VINNOVA and runs from 2014 to 2017.
The three working packages for PiiA-bio are:
- Package 1 - Optimal control of industrial fed-batch fermentation process.
- Package 2 - Optimal control of separation stages.
- Package 3 - Automation of a small-scale drug production platform.
Package 1 - Optimal control of industrial fed-batch fermentation process
The topic of Package 1 is bioprocess control, more specifically control of industrial-sclae microbial fed-batch bioprocesses. Its focus is therefore on methods which are easy to implement in an industrial setting, which gives certain limitations on sensors, actuators, and control systems. The main part of the work concerns control of the microbial substrate uptake rate by manipulation of the feed rate of liquid substrate to the bioprocess. Due to large batch-to-batch variations and the complexity of the processes, model-based control can be difficult to use in this type of system. The approach used in this work circumvents this problem by utilizing perturbations in the feed rate. It has previously been shown that the metabolic state, with regard to substrate uptake rate, can be determined by analyzing the perturbation response in the dissolved oxygen level of a microbial process.
The concept has been tested experimentally in industrial pilot and production scale. It has been demonstrated that a controller based on this concept can be used to compensate for batch-to-batch variations in feed demand and can rapidly compensate for changes in the demand. It has also been shown that the method can be used for monitoring and control in bioprocesses with a volume over 100 m3, using a low-complexity estimation algorithm suited for industrial use.
 Pilot plant at Novozymes
Pilot plant at Novozymes
Package 2 - Optimal control of separation stages
Package 2 studies different steering strategies during chromatographic separation of biopharmaceutical drugs. Three particularly interesting strategies are implemented on a laboratory scale.
a) Pool steering: Steering strategies for fractioning of the outflow from a column, in product and non-product. Pooling is the decision regarding at what times a valve should open and close. The flow passing the valve during this certain time will determine product quantity and quality, i.e. determine the performance of the separation stage.
b) Separation steering: The separation during a separation stage is determined by the structure and amount of raw material loaded into the column, as well as the conditions at elusion. Often, this means that the following batch can be steered by the behavior of the previous batch.
c) Self-optimizing control: By using measurements of inflow and outflow, important process parameters can be estimated. This estimation further becomes the base for an optimization of the following batch. After a number of batches, the steering system is able to independently find the optimal point for all parameters.
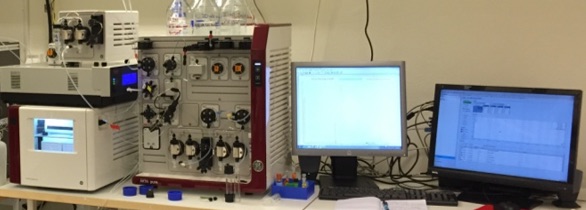 Implementation of Package 2
Implementation of Package 2
Package 3 - Automation of small-scale drug production platform
Package 3 is focused on control and automation of a prototype on a lab-scale platform for production. The aim is to make the system as autonomous as possible. The system consists of a modified version of a regular separation system in a lab-scale, and ÄKTA Pure by GE Healthcare, modified so that several separation stages can be run consecutively on the same equipment (see image).

Several separation stages running on the same equipment
Project Results
- Validated method for optimal fed-batch control in full-scale industrial bioprocess.
- Methods and metodology for optial control of proetin separation steps.
- Archtecture of an automation system for lab-scale protein production.

Pilot plant at Novozymes
 Implementation of Package 2
Implementation of Package 2